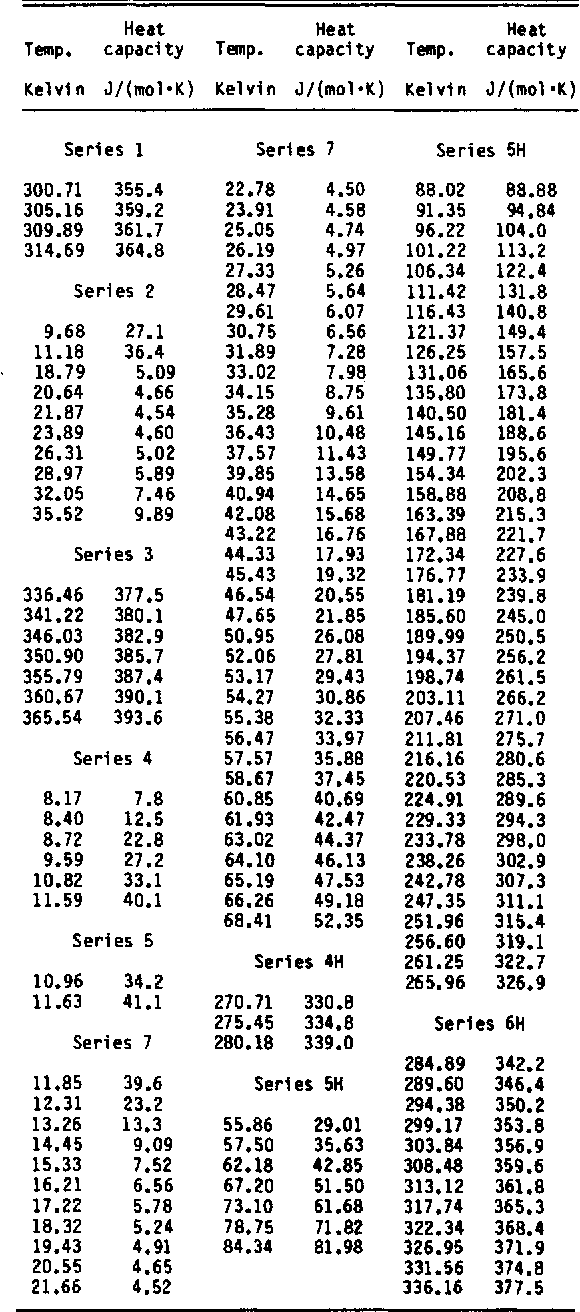
Heat capacity and thermodynamic properties of andradite garnet, Ca3Fe2Si3O12, between 10 and 1000 K and revised values for ΔfGom (298.15 K) of hedenbergite and wollastonite
@article{Robie1987HeatCA,
title={Heat capacity and thermodynamic properties of andradite garnet, Ca3Fe2Si3O12, between 10 and 1000 K and revised values for $\Delta$fGom (298.15 K) of hedenbergite and wollastonite},
author={Richard A. Robie and Zhao Bin and Bruce S. Hemingway and Mark D. Barton},
journal={Geochimica et Cosmochimica Acta},
year={1987},
volume={51},
pages={2219-2224},
url={https://api.semanticscholar.org/CorpusID:98188098}
}28 Citations
Heat capacities of synthetic hedenbergite, ferrobustamite and CaFeSi2O6 glass
- 1987
Materials Science, Physics
Almandine: Lattice and non-lattice heat capacity behavior and standard thermodynamic properties
- 2012
Materials Science
Abstract The heat capacity of three synthetic polycrystalline almandine garnets (ideal formula Fe3Al2Si3O12) and one natural almandine-rich single crystal was measured. The samples were characterized…
Heat capacity and entropy behavior of andradite: a multi-sample and −methodological investigation
- 2018
Geology, Materials Science
Andradite, ideal end-member formula Ca3Fe 3þ 2Si3O12, is one of the common rock-forming garnets found in the Earth’s crust. There are several outstanding questions regarding andradite’s thermodynamic…
Thermodynamics of (Fe2+, Mn2+, Mg, Ca)3− Al2Si3O12 garnet: a review and analysis
- 1999
Chemistry
SummaryThe thermodynamic properties of garnets in the system (Fe2+, Mn2+, Mg, Ca)3A12Si3O12 are reviewed. The thermodynamic properties of the three end-member garnets pyrope, almandine and grossular,…
Modeling the thermodynamic parameters of six endmember garnets at ambient and high pressures from vibrational data
- 2006
Materials Science, Physics
Raman and infrared spectroscopic data at ambient and high pressures were used to compute the lattice contribution to the heat capacities and entropies of six endmember garnets: pyrope, almandine,…
A calorimetric investigation of spessartine: Vibrational and magnetic heat capacity
- 2009
Materials Science, Physics
Single-crystal elasticity of andradite garnet to 11 GPa
- 2004
Materials Science
The high-pressure elastic properties of single-crystal andradite garnet Ca3Fe 3+ Si3O12 were determined by Brillouin scattering to 11 GPa. The pressure dependence of the elastic stiffness constants…
Thermodynamic properties of andradite and application to skarn with coexisting andradite and hedenbergite
- 1991
Materials Science, Geology
AbstractThe enthalpy of formation of andradite (Ca3Fe2Si3O12) has been estimated as-5,769.700 (±5) kJ/mol from a consideration of the calorimetric data on entropy (316.4 J/mol K) and of the…
Grossular: A crystal-chemical, calorimetric, and thermodynamic study
- 2012
Materials Science, Chemistry
Abstract In spite of the amount of research that has been done on grossular, Ca3Al2Si3O12, there is still uncertainty regarding its exact thermodynamic properties. Because of insufficient sample…
Estimating the thermodynamic properties ( AGP and AIl 8 ) of silicate minerals at 298 K from the sum of polyhedral contributions
- 2007
Geology
Many physical properties of silicate minerals can be modeled as a combination of basic polyhedral units (Hazen, 1985, 1988). It follows that their thermodynamic properties could be modeled as the sum…
33 References
Low-temperature molar heat capacities and entropies of MnO2 (pyrolusite), Mn3O4 (hausmanite), and Mn2O3 (bixbyite)
- 1985
Materials Science, Geology
Heat capacities of synthetic hedenbergite, ferrobustamite and CaFeSi2O6 glass
- 1987
Materials Science, Physics
The low-temperature stability of andradite in C-O-H fluids
- 1978
Physics
The low-temperature stability of andradite was experimentally investigated as a function of temperature, Xs6,andf6, at constant P.,ra of2000 bars. Experimental results indicate that the…
Enthalpy of formation of some lime silicates by high-temperature solution calorimetry, with discussion of high pressure phase equilibria
- 1978
Materials Science
Elasticity of uvarovite and andradite garnets
- 1986
Materials Science
The adiabatic single-crystal elastic properties of synthetic end-member uvarovite garnet (Ca3Cr2Si3O12) and a natural sample of 96% andradite garnet (Ca3Fe2Si3O12) have been measured at ambient…
The f O2 -T and f S2 -T stability relations of hedenbergite and of hedenbergite-johannsenite solid solutions
- 1982
Geology
Clinopyroxene in mineralized skarns appears to place local chemical controls on ore mineral precipitation by entering into oxygen- and sulfur-buffering reactions in which garnet is an end product.…
Thermodynamic tabulations for selected phases in the system CaO‐Al2O3‐ SiO2‐H2 at 101.325 kPa (1 atm) between 273.15 and 1800 K
- 1981
Materials Science
The standard thermodynamic properties of phases in the lime‐alumina‐silica‐ water system between 273.15 and 1800 K at 101.325 kPa (1 atm) were evalated from published experimental data. Phases…
The Crystal Chemistry of the Silicate Garnets
- 1971
Materials Science, Chemistry
Refined structures of eight natural garnets [Cr-pyrope, almandine, spessartine, Mngrossular, grossular, uvarovite, goldmanite, and andradite] are compared with that of synthetic pyrope to determine…
A differential thermal analysis study of the high-temperature polymorphism of calcite at high pressure
- 1976
Geology
A DTA-investigation has been conducted in the stable field of calcite between 8 to 45 kb and 400 to 1300 ° C. Two high temperature transitions of calcite with moderate pressure dependency were…